- bd@jeevanscientific.com
- +91-40-6736 4700
History & Milestones
2021
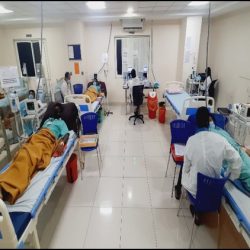
COMPLETED FIRST GLUCOSE CLAMPING STUDY: MAR 2021

SUCCESSFUL COMPLETION OF NABL INSPECTION MAR 20 – MAR 21, 2021
Our Clinical path lab has been assessed and accredited in accordance with the standard ISO 15189:2012 "Medical laboratories - Requirements for quality and competence"

MHRA BE INSPECTION 23 - 30 NOV 2021
Successful Completion of MHRA BE Inspection (with NO Critical Observations)
COMPLETED FIRST GLUCOSE CLAMPING STUDY: MAR 2021
Despite of global COVID-19 pandemic challenges we successfully completed our first Glucose clamping study
SUCCESSFUL COMPLETION OF NABL INSPECTION MAR 20 – MAR 21, 2021
Our Clinical path lab has been assessed and accredited in accordance with the standard ISO 15189:2012 "Medical laboratories - Requirements for quality and competence"
MHRA BE INSPECTION 23 - 30 NOV 2021
Successful Completion of MHRA BE Inspection (with NO Critical Observations)
USFDA INSPECTION 20 JAN – 24 JAN 2020
Successfully completed USFDA inspection with no 483
GLUCOSE CLAMPING UNIT JUN 2020
Jeevan Scientific has established an exceptional and complex service portfolio of GLUCOSE CLAMPING TECHNIQUE PHASE I UNIT
CDSCO INSPECTION 03 DEC – 04 DEC 2019
Successfully completed CDSCO renewal audit. Received renewal approval to our clinical & analytical facilities.
SUCCESSFULLY COMPLETED OUR 1ST PMW STUDY OCT 2019
Successfully completed 1st Post menopausal women subject Pivotal BA/BE study.
USFDA INSPECTION 15 APR - 24 APR 2019
Successfully completed USFDA inspection with no 483.
WHO INSPECTION 04 MAR - 07 MAR 2019
Successfully completed WHO inspection without any major observations
200 BE STUDIES - FEB 2019
Reached mark of 200 studies in a span of 2 years from the date of initiation of our first full scope BE study in Feb 2017
JAN 09 -12, 2018 USFDA INSPECTION
Successfully completed our First USFDA inspection to our Clinical Facility
GLUCOSE CLAMPING UNIT JUN 2020
Initiated our First full scope BE study
JUN 05 - 08, 2017
Successfully completed our First USFDA inspection to Bio-analytical facility. Concluded with no 483.
OCT 2016
Inauguration Jeevan Scientific's New Clinical Facility with 132 bed capacity in Balanagar - Hyderabad
DEC 2016
Clinical facility inspected and approved by CDSCO
MAR 2015
Bio-analytical facility inspected and approved by CDSCO
APR 2014
Dr. Rajendra Prasad has joined as a CEO & Executive Director
NOV 2014
Listed in BSE
DEC 2014
Inauguration of Jeevan Scientific New Corporate and Bio-analytical facility
MEDICAL WRITING SERVICES
Started Medical Writing Services
2001
Listed in Bangalore Stock Exchange
1999
Incorporation of Jeevan Softech Pvt Ltd
2020

USFDA INSPECTION 20 JAN – 24 JAN 2020
Successfully completed USFDA inspection with no 483
GLUCOSE CLAMPING UNIT JUN 2020
Jeevan Scientific has established an exceptional and complex service portfolio of GLUCOSE CLAMPING TECHNIQUE PHASE I UNIT
2019

CDSCO INSPECTION 03 DEC – 04 DEC 2019
Successfully completed CDSCO renewal audit. Received renewal approval to our clinical & analytical facilities.

SUCCESSFULLY COMPLETED OUR 1ST PMW STUDY OCT 2019
Successfully completed 1st Post menopausal women subject Pivotal BA/BE study.

USFDA INSPECTION 15 APR - 24 APR 2019
Successfully completed USFDA inspection with no 483.

USFDA INSPECTION 15 APR - 24 APR 2019 WHO INSPECTION 04 MAR - 07 MAR 2019
Successfully completed WHO inspection without any major observations

200 BE STUDIES - FEB 2019
Reached mark of 200 studies in a span of 2 years from the date of initiation of our first full scope BE study in Feb 2017
2018

JAN 09 -12, 2018 USFDA INSPECTION
Successfully completed our First USFDA inspection to our Clinical Facility
2017

GLUCOSE CLAMPING UNIT JUN 2020
Initiated our First full scope BE study

JUN 05 - 08, 2017
Successfully completed our First USFDA inspection to Bioanalytical facility. Concluded with no 483.
2016

OCT 2016
Inauguration Jeevan Scientific's New Clinical Facility with 132 bed capacity in Balanagar- Hyderabad

DEC 2016
Clinical facility inspected and approved by CDSCO
2015

MAR 2015
Bioanalytical facility inspected and approved by CDSCO
2014

APR 2014
Dr. Rajendra Prasad has joined as a CEO & Executive Director

NOV 2014
Listed in BSE

DEC 2014
Inauguration of Jeevan Scientific New Corporate and Bioanalytical facility
2011

MEDICAL WRITING SERVICES
Started Medical Writing Services
2001

2001
Listed in Bangalore Stock Exchange
1999

1999
